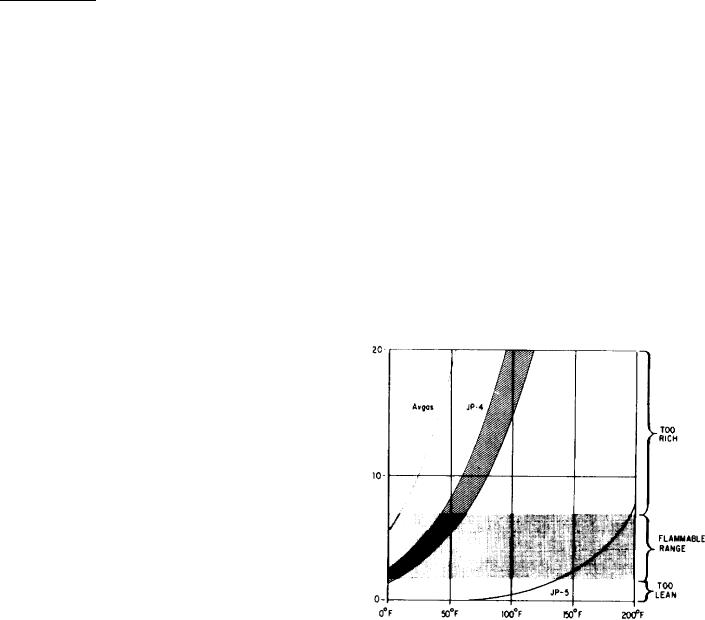
Volatility
developed to meet specific operating or handling
characteristics. A study of the basic characteristics
Volatility measures the ability of a liquid to
of turbine fuels will help you understand the
convert to a vaporous state. Fuel must vaporize
importance of delivering the proper fuel to the
and the vapor be mixed in a given percentage of
aircraft. Such a study is also valuable in
air for it to burn or explode. Only fuel-air
understanding the need for safety and caution in
mixtures within the flammable range will burn
handling these fuels. This section includes the
(fig. 4-1). Volatility of a fuel effects starting,
basic characteristics of engine fuels.
range, and safety. A highly volatile fuel starts
easier (especially at low temperatures or under
CHARACTERISTICS
adverse conditions) and has less range (due to fuel
evaporation in flight). The fuel has a higher
tendency to vapor lock and is more susceptible
Aircraft engine fuels are petroleum products
to a fire during a crash. The volatility of a
manufactured from crude oil by oil refineries.
petroleum fuel is usually measured in terms of
They are classified as inflammable liquids.
vapor pressure and distillation.
Any material easily ignited that burns rapidly is
inflammable. (NOTE: The terms flammable and
The vapor pressure shows the tendency to
vaporize at specific temperatures. Vapor pressure
inflammable mean the same.) Under proper
is measured in a Reid Vapor Pressure Test Bomb.
conditions, fuel can explode with force similar to
In the test, one volume of fuel and four volumes
dynamite. Death can result if the vapors of fuel
of air are contained in a sealed bomb fitted with
are inhaled in sufficient quantities. Serious skin
a pressure gauge. The container and fuel are
irritation can result from contact with the fuel in
heated to 100F, shaken, and then you read the
the liquid state. In liquid form, aircraft fuels are
pressure on the gauge. The pressure shown on the
lighter than water, and in vapor form they are
gauge is known as the Reid Vapor Pressure (RVP)
heavier than air. Consequently, water in the fuel
usually settles to the bottom of the container. And
and is expressed in pounds per square inch (psi).
vapors of these fuels, when released in the air,
The higher the pressure the more volatile the fuel.
tend to remain close to the ground, thus increasing
The distillation measurement for volatility
the danger to personnel and property. From safety
measures the amount of fuel boiled off at specific
temperatures. Since turbine fuels are a mixture
and health standpoints, aircraft engine fuels must
be handled with caution.
of hydrocarbons (gasoline and kerosene), they
have a wide range of boiling points. This
In the selection of a fuel, several factors must
test records the boiling ranges. The military
be considered. Since one fuel cannot have all the
requirements to the greatest degree, the fuel
selected is a compromise of various factors.
Specific properties of fuels are determined
through testing. These tests determine the
volatility, density, heating value, combustion,
safety, and handling characteristics of the fuels.
There are hundreds of test that determine the
physical, chemical, and performance properties
of fuel. We limit this discussion to the most
common and important ones as follows:
1. Volatility (vapor pressure and distillation
2. Flash point and fire point
3. Heat energy content
4. Viscosity
5. Handling characteristics
6. Combustion products
7. Effects of additives and impurities
Figure 4-1.-Vaporization of aviation fuels at atmospheric
pressure.
8. Freeze point
4-2

