PASCAL'S LAW: Pressure exerted at any
these observations he concluded that for a
point upon an enclosed liquid is transmitted
constant temperature the product of the volume
undiminished in all directions.
and pressure of an enclosed gas remains constant.
This conclusion became Boyle's law.
B E R N O U L L I ' S PRINCIPLE: If an
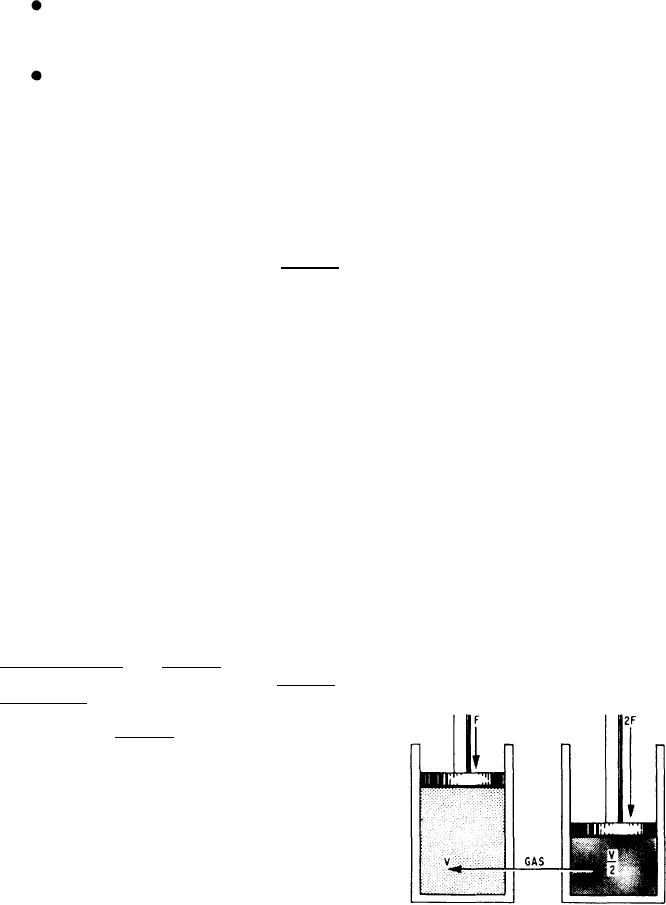
You can demonstrate Boyle's law by confining
incompressible fluid flowing through a tube
a quantity of gas in a cylinder that has a tightly
reaches a constriction, or narrowing of the
fitted piston. Apply force to the piston so as to
tube, the velocity of fluid flowing through the
compress the gas in the cylinder to some specific
constriction increases and the pressure decreases.
volume. If you double the force applied to the
piston, the gas will compress to one half its
original volume (fig. 4-9).
Charles's Law
Changes in the pressure of a gas also affect
the density. As the pressure increases, its volume
Jacques Charles, a French scientist, provided
decreases; however, there is no change in the
much of the foundation for the modern kinetic
weight of the gas. Therefore, the weight per unit
theory of gases. He found that, if the pressure
volume (density) increases. So it follows that the
is held constant, all gases expand and contract in
density of a gas varies directly with the pressure if
direct proportion to the change in the absolute
the temperature is constant.
temperature. Any change in the temperature of
a gas causes a corresponding change in volume.
Newton's Laws
Therefore, if a given sample of a gas were heated
while confined within a given volume, the pressure
Sir Isaac Newton was an English philosopher
should increase. Actual experiments found that
and mathematician who lived from 1642 to 1727
for each 1C increase in temperature, the increase
A.D. He was the formulator of the basic laws of
in pressure was about l/273 of the pressure at
modern philosophy concerning gravity and
0C. Thus, it is normal practice to state this
motion.
relationship in terms of absolute temperature. In
equation form, this part of the law becomes
NEWTON'S FIRST LAW.--Newton's first
law states that a body at rest tends to remain at
P1T2 = P2T1,
rest. A body in motion tends to remain in
motion. This law can be demonstrated easily in
where
everyday use. For example, a parked automobile
will remain motionless until some force causes it
P = pressure, and
to move-a body at rest tends to remain at rest.
The second portion of the law-a body in
T = temperature.
motion tends to remain in motion-can be
demonstrated only in a theoretical sense. The
In words, this equation states that with a
same car placed in motion would remain in
constant volume, the absolute pressure of an
enclosed gas varies directly with the absolute
temperature. Remember, when using this for-
mula, you must convert stated pressures and
temperatures to absolute values.
Boyle's Law
Compressibility is an outstanding charac-
teristic of gases. Robert Boyle, an English
scientist, was among the first to study this
characteristic. He called it the "springiness" of
air. He discovered that when the temperature of
an enclosed sample of gas was kept constant and
the pressure doubled, the volume was reduced to
half the former value. As the applied pressure was
Figure 4-9.--Gas compressed to half its original volume by
decreased, the resulting volume increased. From
a double force.
4-9

