temperature. For every 10-degree difference in
plates are insulated from each other by suitable
temperature above 80F, you must add 0.004 to the
separators (wood, rubber, or glass) and are submerged
specific gravity reading. For every 10-degree difference
in a sulfuric acid solution (electrolyte).
in temperature below 80F, you must subtract 0.004.
RATING
For example, suppose you get a hydrometer reading of
1.280 from a cell. Checking the temperature of the
Lead-acid batteries are rated by their voltage
electrolyte with a thermometer, you get a reading of
and ampere-hour capacity. Standard automotive
60F. This is a 20-degree difference from the normal
batteries have a 20-hour discharge rate, which is equal
temperature of 80F. Therefore, you must subtract
to the constant current in amperes that the battery can
0.008 from the hydrometer reading of 1.280 to get the
supply continuously for 20 hours before the voltage
true specific gravity of the battery, which is 1.272.
drops to a specified low-voltage level. For example,
Note that a 20-degree difference in temperature did
a battery rated by the manufacturer as 12-volts,
not make a significant difference in the specific gravity
280-ampere-hours can discharge at a rate of 14 amperes
(less than 1 percent). From this you can see that it takes
for 20 hours before the voltage drops to its specified
a relatively large difference in temperature to make a
limiting voltage (280 ampere-hours 14 amperes = 20
significant difference in specific gravity. Remember,
hours). The voltage and ampere-hour rating of a battery
however, that it is the temperature of the electrolyte that
is usually stamped on the battery case.
is important, not the temperature of the surrounding air.
Table 6-1.--Specific Gravity of Lead-Acid Batteries
If the vehicle has been running, the battery is likely to
be warmer that the surrounding air. Likewise, if the
SPECIFIC GRAVITY
STATE OF CHARGE
vehicle has not been running, the battery may be cooler
than the surrounding air. In extremely hot or extremely
1.265-1.290
Fully charged battery
cold conditions, readings may not be accurate even
1.235-1.260
3/4 charged
after correcting for differences in temperature.
1.205-1.230
1/2 charged
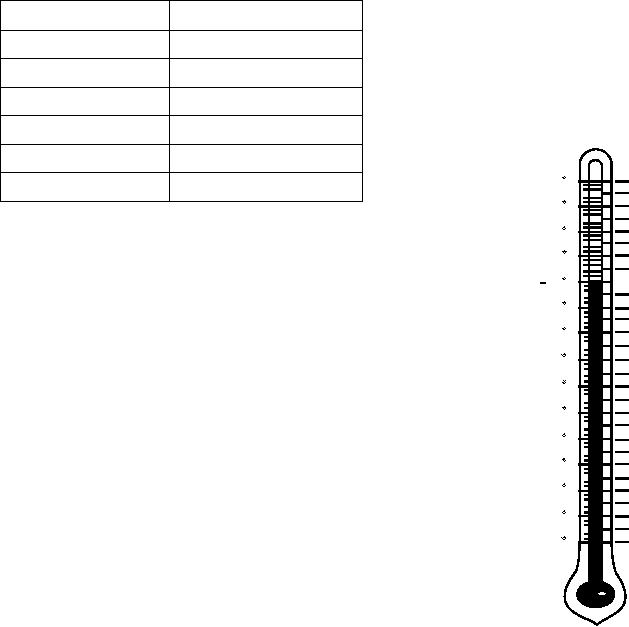
A specific gravity correction chart, similar to the
one shown in figure 6-10, is normally found on the
1.170-1.200
1/4 charged
1.140-1.165
Barely operative
+.016
120
1.100-1.135
Completely discharged
+.014
+.012
110
+.010
As a lead-acid battery discharges, the sulfuric acid
+.008
100
+.006
is absorbed by the plates and the electrolyte is gradually
+.004
90
converted into water. This action provides a guide in
+.002
0
NORMAL 80
determining the state of discharge of the lead-acid cell.
-.002
All that is necessary to determine the state of charge of
-.004
70
-.006
a battery is to determine the amount of sulfuric acid
-.008
60
remaining in the electrolyte. This is easily done with a
-.010
-.012
50
hydrometer. The hydrometer measures specific gravity,
-.014
which is an accurate indication of the percentage of
-.016
40
-.018
sulfuric acid remaining in the electrolyte. Table 6-1 lists
-.020
30
specific gravity readings of lead-acid batteries in
-.022
20
-.024
various states of charge.
-.026
-.028
10
In taking readings of specific gravity, the
-.030
temperature must be taken into account. Specific
-0
-.032
-.034
gravity is a measure of the density of the electrolyte,
-.036
-10
and electrolyte becomes less dense as its temperature
-.038
-.040
-20
rises, and more dense as its temperature falls.
A hydrometer is marked (calibrated) to read
specific gravity at a temperature of 80F. Hydrometer
readings taken on electrolyte that is warmer or colder
ASf06010
than 80F must be corrected to account for difference in
Figure 6-10.--Specific gravity temperature correction chart.
6-10

