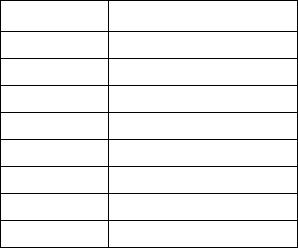
other substances are expressed as decimals. Table 11-1
the temperature of the substance will rise. If heat is
shows examples of the specific heat of some sub-
taken away from a substance, the temperature of the
stances.
substance will decrease.
Table 11-1.--Specific Heat
In discussing heat, we need to make a distinction
between intensity and quantity. It will help to compare a
spoonful of hot water to a pailful of warm water. The
Material
Specific Heat (Btu/lb)
spoonful of hot water contains a greater intensity of
Water
1.000
heat, but the warm water in the pail possesses a greater
Ice
.504
quantity of heat, because of its greater mass.
Wood
.327
Intensity of Heat
Iron
.129
Intensity of heat is measured by the ordinary
Copper
.095
thermometer with which everyone is familiar. The two
Glass
.187
methods of dividing and numbering the thermometer
Mercury
.033
scales in common use are the Fahrenheit scale and the
Centigrade scale. (Another scale not so commonly
Alcohol
.615
used, except by scientists, is the Kelvin scale.)
Thermal Capacity
Quantity of Heat
Thermal capacity is closely related to specific heat.
The quantity of heat possessed by a substance is
The specific heat of a substance is the number of Btu
measured in terms of the British thermal unit (Btu). A
necessary to raise the temperature of 1 pound of the
Btu is the quantity of heat required to raise the
substance 1 degree Fahrenheit. The thermal capacity of
temperature of 1 pound of pure water 1 degree
a substance is the amount of heat required to raise the
Fahrenheit at or near 39.1F. This is the temperature at
temperature of its whole weight 1 degree. Hence,
which water is at maximum density. For example, to
thermal capacity equals the specific heat of a substance
raise the temperature of 5 pounds of water from 39F to
multiplied by its weight. Thermal capacity may be said
49F requires 50 Btu (5 lb 10F = 50 Btu). For all
to express the total capacity of a given quantity of a
practical purposes, the Btu is considered constant
substance for absorbing and storing heat. Thermal
between 32F and 212F, though it does vary a slight
capacity is stated, not as a ratio, but as a certain number
amount.
of Btu.
TYPES OF HEAT
Sensible Heat
For a full understanding of the principles of air
Heat that is added to, or subtracted from, a
conditioning, you must understand the terminology of
substance that changes its temperature but not its
the science. One aspect of that is the way in which heat
physical state is called sensible heat. Sensible heat is
is classified. We have already discussed intensity of
the heat that can be indicated on a thermometer. For
heat, which is measured by a thermometer, and quantity
example, if you heat a cup of water from 50F to 70F,
of heat, which is measured in Btu. The different types
its temperature increases 20 degrees but the water stays
of heat are discussed here.
in its liquid state.
Specific Heat
Sensible heat is the heat that human senses can also
react to, at least within certain ranges. For example, if a
Specific heat is the number of Btu that must be
person puts his finger into a cup of water, his senses
added to a unit weight of substance to raise its
readily tell him whether it is cold, cool, tepid, hot, or
temperature 1 degree Fahrenheit. (The unit weight is
very hot. Human senses are not sufficiently
normally considered to be 1 pound.) Technically, the
discriminating to give precise information about the
specific heat of a substance is the ratio of the amount of
extreme temperatures of ice and steam or other
heat required to change the temperature of the same
substances having temperatures beyond the range of
weight of water 1 degree. Since the specific heat of
human sensory mechanisms. Ice merely seems cold and
water is, by definition, equal to 1, the specific heat of
steam seems hot whatever their temperatures.
11-2

