nonflammable, but it will support combustion when
combined with other gases. This means that it aids in
burning, and this burning gives off considerable heat and
light. In its free state, oxygen is one of the most common
elements. The atmosphere is made up of approximately
21 parts of oxygen and 78 parts of nitrogen, with the
remainder being rare gases. It is the presence of oxygen
in the air that causes rusting of ferrous metals, the
discoloration of copper, and corrosion of aluminum.
This action is known as oxidation.
Oxygen is obtained commercially either by the
liquid air process or by the electrolytic process. In the
liquid air process, air is compressed and cooled to a point
where the gases become a liquid. As the temperature of
the liquid air is raised, nitrogen in a gaseous form is
given off first, since its boiling point is lower than that
of liquid oxygen. These gases, having been separated,
are further purified and compressed into cylinders for
use.
In the electrolytic process, water is broken down
into hydrogen and oxygen by the passage of an electric
current through it. The oxygen collects at the positive
terminal and the hydrogen at the negative terminal. Each
of the gases is then collected and compressed into
cylinders for use.
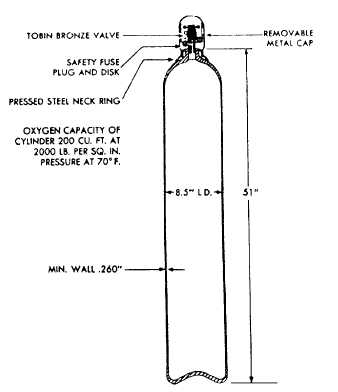
OXYGEN CYLINDERS.—A typical oxygen
cylinder (fig. 15-24) is made of steel and has a capacity
of 220 cubic feet at a pressure of 2,000 psi and a
temperature of 70°F. Each oxygen cylinder has a
high-pressure outlet valve located at the top of the
cylinder, a removable metal cap for the protection of the
outlet valve during shipment or storage, and a low
melting point safety fuse plug and disk. All oxygen
cylinders are painted green for identification. Technical
oxygen cylinders are solid green, while breathing
oxygen cylinders are green with a white band around the
top.
CAUTION
Oxygen should never be brought in contact
with oil or grease. In the presence of pure
oxygen, these substances become highly
combustible. Oxygen hose and valve fittings
should never be oiled or greased or handled
with oily or greasy hands. Even grease spots
on clothing may flare up or explode if struck
by a stream of oxygen.
PRESSURE REGULATORS.—The gases
compressed in oxygen and acetylene cylinders are at
pressures too high for oxyacetylene welding. Regulators
Figure 15-24.—Typical oxygen cylinder.
are necessary to reduce pressure and control the flow of
gases from the cylinders. Most regulators in use are
either the single-stage or the two-stage type. Single-
stage regulators reduce the pressure of the gas in one
step; two-stage regulators do the same job in two steps
or stages. Generally, less adjustment is necessary when
two-stage regulators are used.
Figure 15-25 shows a typical single-stage regulator.
The regulator mechanism consists of a nozzle through
which the high-pressure gases pass, a valve seat to close
off the nozzle, and balancing springs. These are all
enclosed in a suitable housing. Pressure gauges are
provided to indicate the pressure in the cylinder or
pipeline (inlet), as well as the working pressure (outlet).
The inlet pressure gauge, used to record cylinder
pressures, is a high-pressure gauge and is graduated
from 0 to 3,000 psi. The outlet pressure gauge, used to
record working pressures, is a low-pressure gauge and
is graduated from 0 to 500 psi.
In the oxygen regulator, the oxygen enters through
the high-pressure inlet connection and passes through a
glass wool filter that removes dust and dirt. Turn the
adjusting screw in, to the right, to allow the oxygen to
pass from the high-pressure chamber to the low-
pressure chamber of the regulator, through the regulator
outlet, and through the hose to the torch at the pressure
shown on the working pressure gauge. Changes in this
pressure may be made at will, simply by adjusting the
15-18