environment destroying the anodic area. Note that the
surface of a metal may contain anodic and cathodic
areas because impurities or alloying constituents may
have different potentials than the base metal.
Electrochemical attack is evident in several forms.
The form you find depends upon the metal involved,
its size and shape, its specific functions, atmospheric
conditions, and type of corrosion-producing agent
(electrolyte) present.
There are many factors that affect the type, speed,
cause, and the seriousness of metal corrosion. Some of
these factors you can control; others you cannot.
Preventive maintenance factors, such as inspections,
cleaning, painting, and preservation, are within the
control of the operating squadron. They offer positive
means of preventing corrosion.
The electrochemical reaction, which causes metal
to corrode, is more dangerous under wet, humid
conditions than under dry conditions. The salt in
seawater and the salt in the air are the largest single
cause of aircraft corrosion. Hot climates speed the
corrosion process because the electrochemical
reaction develops fastest in a warm solution. The warm
moisture in the air is usually enough to start corrosion
of the metals if they are uncoated. As expected, hot,
dry climates usually provide relief from constant
corrosion problems. Extremely cold climates will
produce corrosion problems when a salt-laden
atmosphere is present. Melting snow or ice provides
the necessary water to begin the electrochemical
reaction.
Thick structural sections are subject to corrosive
attack because of possible variations in their
composition, particularly if they were heat-treated
during fabrication. Similarly, when large sections are
machined or cut out after heat treatment, thinner
sections have different physical characteristics than
the thicker areas. Usually a difference in physical
characteristics provides enough difference in
electrical potential to make the piece highly
susceptible to corrosion. Another factor relating to the
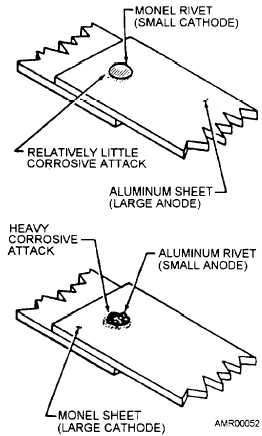
size of materials is the relationship between dissimilar
metals. (See figure 4-2.) If electrical contact develops
between two dissimilar metals, the corrosion attack on
the more active metal or anode (smaller size compared
to the less active one) will be severe and extensive. See
figure 4-2, bottom view. If the area of the less active
metal is small compared to the other, anodic attack will
be slight (fig. 4-2, top view).
Corrosion on avionics equipment is a continuing
process. The equipment does not have to be installed,
operating, or exposed to a particularly harsh
environment to corrode. The rate of the corrosion
process is determined by the temperature, humidity,
and chemicals in the environment. Moisture is the
single largest contributor in avionics corrosion. It
makes little difference whether the moisture is in the
form of vapor or liquid. Its affects are detrimental to
metals.
A clean aircraft retains its aerodynamic efficiency
and safety. Serious damage to the exterior and interior
surfaces of aircraft can result from the lack of correct
information about cleaning materials and equipment
and their use. Shipboard procedures are not
necessarily the same as procedures ashore, but the
same materials are available to produce comparable
results.
A problem you may face when fighting corrosion
is knowing what materials to use, where to find them,
and their limitations. You should use only those
materials that have military specifications. Corrosion
control information can be found in many directives
Figure 4-2.—Effects of area relationships in dissimilar metal
contacts.
4-3