time-consuming, and reduce equipment usage time.
These problems can be avoided through good
preventive maintenance practices and procedures.
To have good preventive maintenance practices
and procedures, you must know and be able to apply
the common types of corrosion prevention and
moisture protecting materials.
Q1. How does corrosion endanger aircraft or reduce
the margin of safety?
Q2. All maintenance personnel must be formally
Q3.
trained in what program?
What is the primary factor to consider when
selecting materials for constructing an aircraft?
on unpainted areas of working parts. Finally, shrouds,
covers, caps, and other mechanical equipment provide
varying degrees of protection from corrosive
mediums. However, none of these procedures will
provide 100-percent protection. Weathering causes
paint to oxidize and decay. Sealants may be worked
out by vibration or be eroded by rain and windblast.
Preservatives offer only temporary protection when
used on operating aircraft. The mechanical coverings
can be installed improperly or negligently.
Control of corrosion begins with an understanding
of the causes and the nature of corrosion. Corrosion is
CORROSION THEORY
in its most familiar form is a reaction between metal
and water, and is electrochemical in nature.
the process of electrochemical or direct chemical
attack on metals. The reaction is similar to that which
occurs when acid is applied to bare metal. Corrosion
LEARNING OBJECTIVES: Define the theory
of corrosion and its process. Identify the
publications and materials used in the
prevention of corrosion.
Metal corrosion is the decay of metals as they
combine with oxygen to form metallic oxides. Corrosion
is a chemical process that is the reverse of the process of
smelting the metals from their ores. Very few metals are
found in their pure state in nature. Most are found as
metallic oxides. These oxides have other undesirable
impurities in them. The refining process involves the
extraction of the base metal from the ore. The base metal
is then mixed with other elements (either metallic or
nonmetallic) to form alloys. Alloying elements are added
to base metals to develop a variety of useful properties.
For instance. in aircraft structural applications, high
strength-to-weight ratios are the most desirable
properties of an alloy.
After the base metals are refined, whether alloyed
or not, they have a potential to return to their natural
state. However, potential is not sufficient in itself to
begin and promote this reversion; a corrosive
environment must also exist. The significant element
of the corrosive environment is oxygen. The process
of oxidation (combining with oxygen) causes wood to
rot or bum and metals to corrode.
Control of corrosion depends upon maintaining a
separation between susceptible alloys and the
corrosive environment. This separation is
accomplished in various ways. A good intact coat of
paint provides most of the corrosion protection on
naval aircraft. Sealants used at seams and joints prevent
entry of moisture into the metal. Preservatives are used
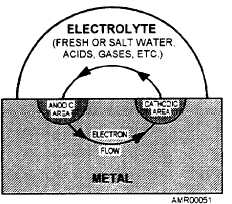
The electrochemical attack involves metals of
different electrical potential. These metals do not have
to be in direct contact. If one metal contains positively
charged ions and the other negatively charged ions, all
that is needed is an electrical conductor. When the
conductor is present, current will flow between the two
metals, as in the discharge of a dry-cell battery. In
electrochemical corrosion, the electrical conductor
may be any foreign material, such as water, dirt,
grease, or any debris that is capable of acting as an
electrolyte. The presence of salt in any of the foregoing
mediums accelerates the current flow and increases the
rate of corrosive attack.
Once an electrical connection is made, the electron
flow is established in the direction of the negatively
charged metal (cathode). This action eventually
destroys the positively charged metal (anode).
Preventive measures include avoiding the
establishment of the electrical circuit and removing
corrosion as soon as possible to avoid serious damage.
Figure 4-1 shows the electron flow in a corrosive
Figure 4-1.—Simplified corrosion cell.
4-2